Strattera Paper Database
Visual Abstract
Atomoxetine treatment of attention-deficit/hyperactivity disorder in young adults with assessment of functional outcomes: a randomized, double-blind, placebo-controlled clinical trial
Atomoxetine for ADHD in Young Adults
September 15, 2024
author
Durell TM, Adler LA, Williams DW, Deldar A, McGough JJ, Glaser PE, Rubin RL, Pigott TA, Sarkis EH, Fox BK
journal
J Clin Psychopharmacol
Date Published
February 2013
Why link to a visual abstract?
What is a visual abstract?
Original
Study Summary
🔬
What They Studied
The study aimed to assess the effectiveness of atomoxetine (Strattera) on ADHD symptoms and functional outcomes in young adults.
💡
What They Found
Atomoxetine was more effective than placebo in reducing ADHD symptoms and improving quality of life and executive functioning in young adults.
📚
What This Means
These results suggest that atomoxetine (Strattera) is effective in treating ADHD symptoms and enhancing quality of life in young adults, in agreement with current evidence highlighting its FDA-approved use for ADHD treatment.
Study Summary
Study Overview
This study aimed to explore the effects of atomoxetine on young adults with ADHD. Researchers found that atomoxetine not only reduced ADHD symptoms but also enhanced the overall quality of life for these individuals. They noted that ADHD can significantly impair various aspects of life, so improvements in quality of life are crucial in clinical settings.
The findings indicate that atomoxetine is generally well tolerated and may offer an effective treatment option for young adults facing ADHD challenges.
The findings indicate that atomoxetine is generally well tolerated and may offer an effective treatment option for young adults facing ADHD challenges.
Abstract: background
Attention-deficit/hyperactivity disorder (ADHD) is associated with significant impairment in multiple functional domains. This trial evaluated efficacy in ADHD symptoms and functional outcomes in young adults treated with atomoxetine.

Impact on Quality of Life
"This trial found that atomoxetine improved not only ADHD symptoms but also quality-of-life outcomes in young adults as measured by the AAQOL-29."
Clinical Relevance
"Given the well-documented dysfunction across many domains of life experienced by young adults with ADHD, this finding of improvement of participants' quality of life may be of particular value to clinicians."
Treatment Evaluation
"This trial evaluated efficacy in ADHD symptoms and functional outcomes in young adults treated with atomoxetine."
Study Summary
Methods
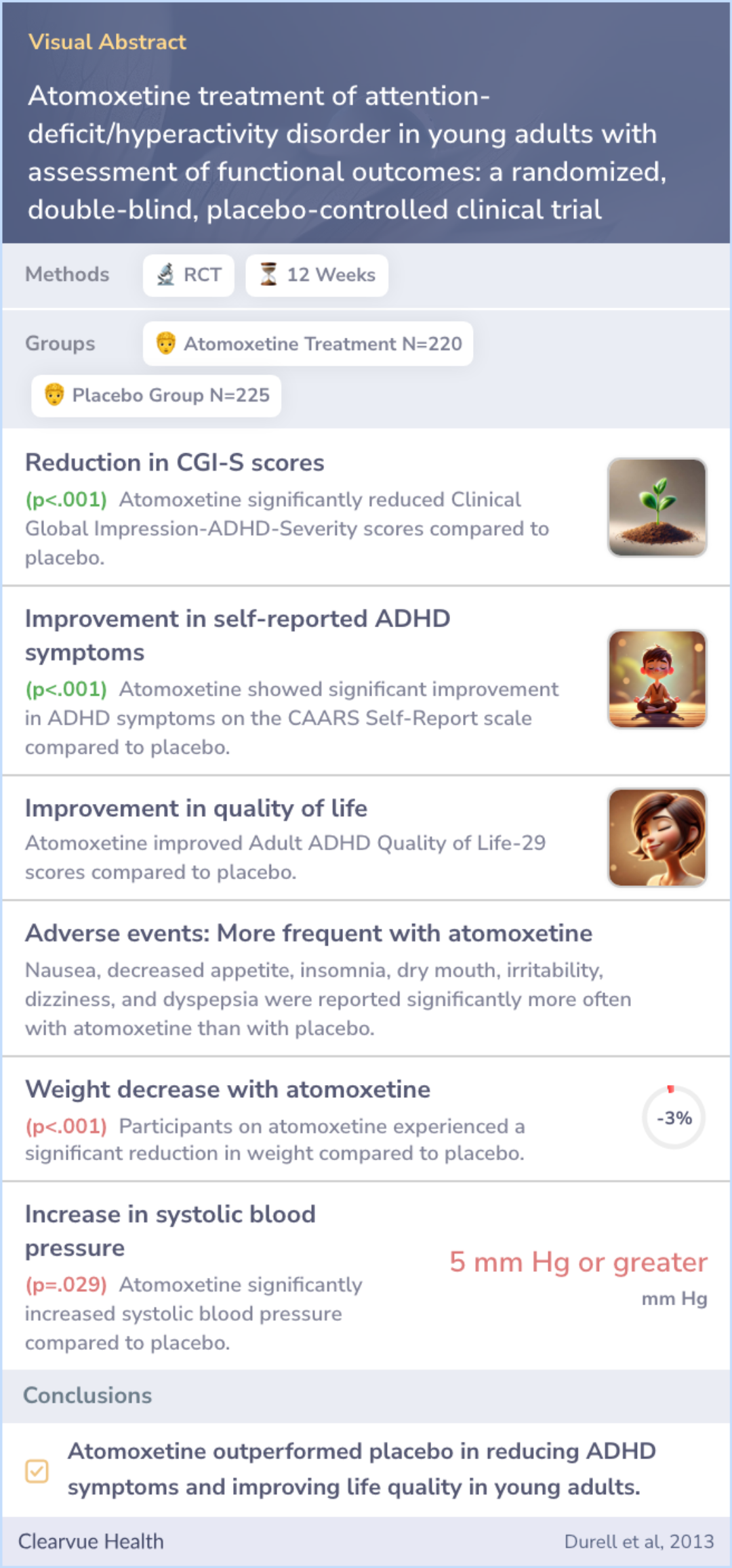
The study involved young adults aged 18-30 who were randomly assigned to receive either atomoxetine (220 participants) or a placebo (225 participants) over a 12-week period. To measure changes in ADHD symptoms, they used a tool called the Conners' Adult ADHD Rating Scale. Researchers also tracked quality of life, overall severity, and improvements as perceived by both doctors and patients.
Other aspects like depression, anxiety, and daily activities were also assessed through various self-report scales to provide a comprehensive view of the participants' progress over the trial.
Other aspects like depression, anxiety, and daily activities were also assessed through various self-report scales to provide a comprehensive view of the participants' progress over the trial.
Abstract: methods
Young adults (18-30 years old) with ADHD were randomized to 12 weeks of double-blind treatment with atomoxetine (n = 220) or placebo (n = 225). The primary efficacy measure of ADHD symptom change was Conners' Adult ADHD Rating Scale (CAARS): Investig...more
Study Summary
Results
The study found that atomoxetine was more effective than the placebo in reducing ADHD symptoms according to the Conners' Rating Scale and helped improve other areas like quality of life and executive functioning. While atomoxetine showed clear benefits in these areas, not all assessments showed significant differences between atomoxetine and placebo.
Adverse effects similar to previous studies of atomoxetine were noted, with common issues like nausea and insomnia being reported more frequently with the medication. Despite this, the medication demonstrated a consistent ability to alleviate major ADHD-related symptoms.
Adverse effects similar to previous studies of atomoxetine were noted, with common issues like nausea and insomnia being reported more frequently with the medication. Despite this, the medication demonstrated a consistent ability to alleviate major ADHD-related symptoms.
Abstract: results
Atomoxetine was superior to placebo on CAARS: Investigator-Rated: Screening Version (atomoxetine [least-squares mean ± SE, -13.6 ± 0.8] vs placebo [-9.3 ± 0.8], 95% confidence interval [-6.35 to -2.37], P < 0.001), Clinical Global Impression-ADHD-Sev...more

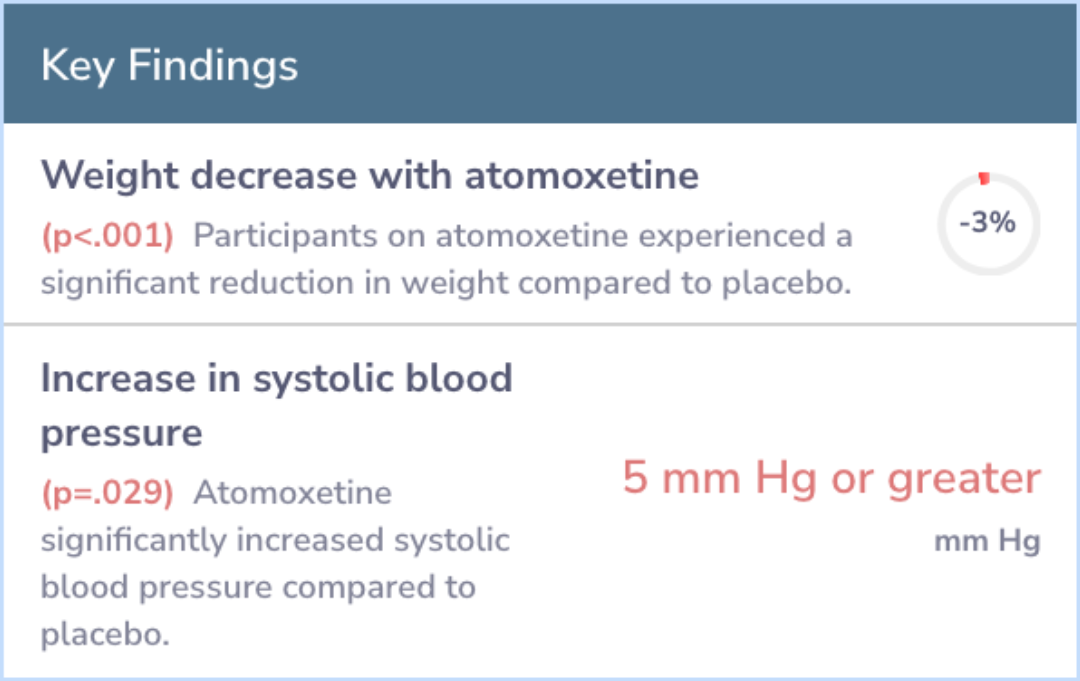
Study Summary
Conclusions
Atomoxetine proved beneficial in alleviating symptoms of ADHD and enhancing quality of life and executive functioning in young adults compared to a placebo. Most participants tolerated the medication well, showing a similar adverse effect profile to earlier research.
The findings suggest atomoxetine can be a valuable treatment option for those struggling with ADHD in this age group, offering both practical benefits and an overall good safety profile.
The findings suggest atomoxetine can be a valuable treatment option for those struggling with ADHD in this age group, offering both practical benefits and an overall good safety profile.
Abstract: conclusions
Atomoxetine reduced ADHD symptoms and improved quality of life and executive functioning deficits in young adults compared with placebo. Atomoxetine was also generally well tolerated.

Background Information
Patient Guide
💊
FDA-Approved for ADHD
Strattera is FDA-approved to treat ADHD in both adults and children over age six.
⚙️
Mechanism: Norepinephrine Inhibitor
Acts by inhibiting norepinephrine reuptake, also affecting dopamine in specific brain regions.
🧬
Pharmacokinetics and Bioavailability
High protein binding and metabolism by CYP2D6; variations exist in extensive vs. poor metabolizers.
🤕
Common Adverse Reactions
Headache, insomnia, nausea, and decreased appetite frequently reported with Strattera use.
❤️
Contraindications: Cardiac Issues
Not recommended for patients with serious cardiac problems or pheochromocytoma.
Professional Guide
Expert Opinion: Atomoxetine for ADHD in Young Adults
The efficacy of atomoxetine in reducing ADHD symptoms and improving quality of life for young adults is well established.
Clinical guidelines underscore its well-tolerated nature, but blood pressure and heart rate monitoring remain advisable.
Atomoxetine's unique profile, differing from stimulants, makes it suitable for adults with a history of substance use, while potential gastrointestinal and sexual side effects need consideration during treatment.
Clinical guidelines underscore its well-tolerated nature, but blood pressure and heart rate monitoring remain advisable.
Atomoxetine's unique profile, differing from stimulants, makes it suitable for adults with a history of substance use, while potential gastrointestinal and sexual side effects need consideration during treatment.
Evidence Summary
Comparing Atomoxetine and Methylphenidate: Exploring Effectiveness and Patient Responses
When comparing Atomoxetine to Methylphenidate, the focus shifts to understanding how each medication performs uniquely in treating ADHD. These medications vary in effectiveness and side effects, reflecting different patient responses. This comparison highlights what patients might experience with each option, offering insights on making informed treatment decisions.
Evidence Summary
Comparing ADHD Medications: Effectiveness and Tolerance
The article explores ADHD medications by assessing their effectiveness and how patients handle side effects. It reviews multiple studies to present a snapshot of treatment safety and outcomes.
Key points include the evaluation of different medications in terms of how well they work and patient tolerance.
The review provides an overview of how various treatments compare, helping to illuminate the broader landscape of ADHD medication outcomes.
Key points include the evaluation of different medications in terms of how well they work and patient tolerance.
The review provides an overview of how various treatments compare, helping to illuminate the broader landscape of ADHD medication outcomes.
Evidence Summary
Adderall XR vs. Strattera: Impacts on Kids with ADHD
Exploring how Adderall XR and Strattera impact children with ADHD shows their distinct effects on attention and behavior in school. Each medication has a unique profile, leading to varying improvements and side effects. Strattera explores this in young adults too, but here it's all about the school kids.
The article reveals differences in side effects, helping parents and educators grasp what to expect from each option for managing ADHD in young learners.
The article reveals differences in side effects, helping parents and educators grasp what to expect from each option for managing ADHD in young learners.
Evidence Summary
Exploring the Multifaceted Nature of ADHD
ADHD is not just one-size-fits-all; it's a mix of different challenges. This study reveals diverse cognitive and emotional deficits in children, using tasks to test memory, response time, and emotional control. Differences were mainly noted in executive functions and reaction time, except in recognizing disgust, pointing to ADHD's varied nature.
Emotional functioning emerges as a distinct element, influencing ADHD beyond typical symptoms like delay aversion.
Emotional functioning emerges as a distinct element, influencing ADHD beyond typical symptoms like delay aversion.