Strattera
Evidence Based Answers
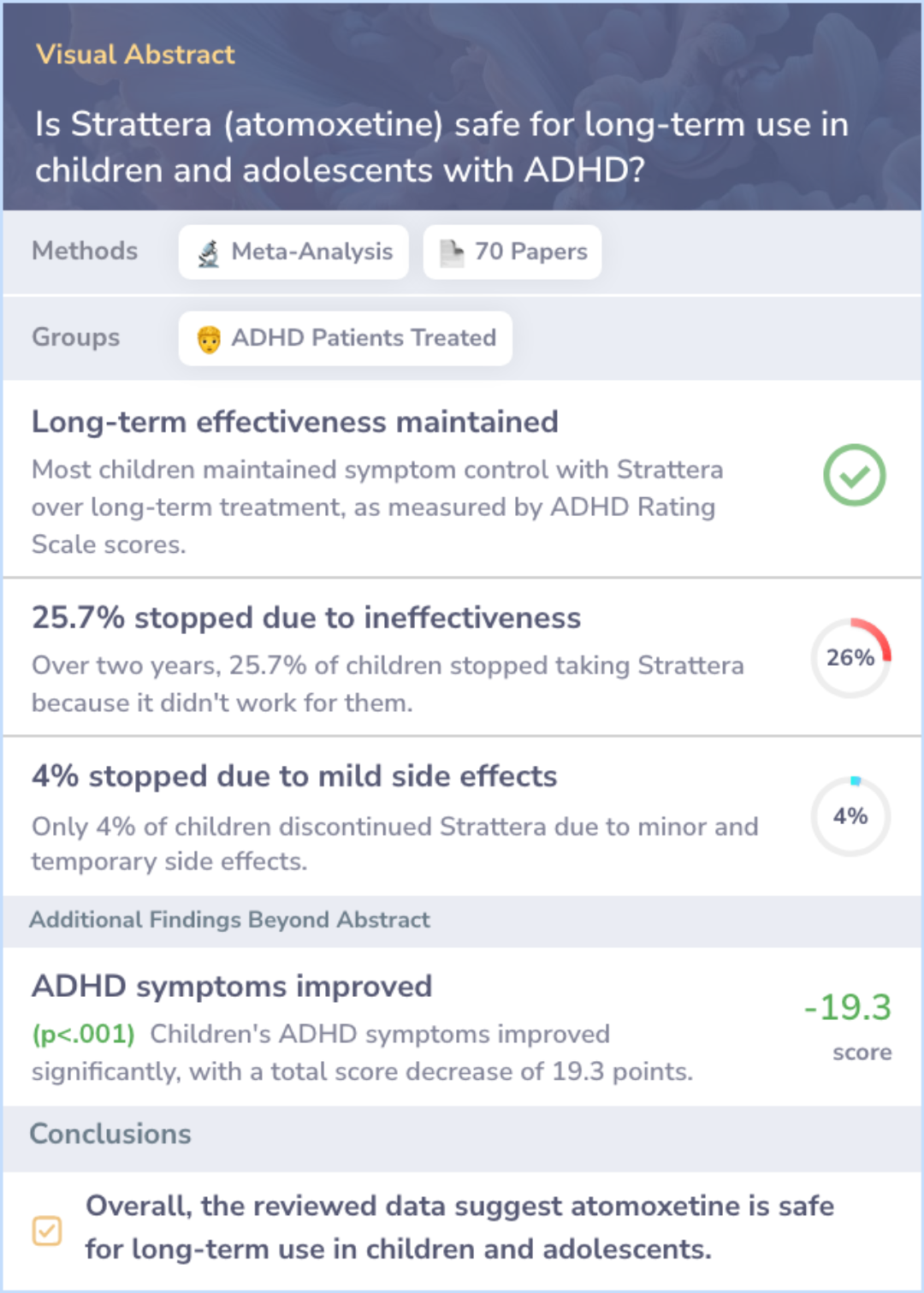
3 Studies: Is Strattera (atomoxetine) safe for long-term use in children and adolescents with ADHD?
Studies Summary
📊
Effectiveness and Growth Impact of Strattera
Strattera is effective for ADHD over the long-term, but less so compared to stimulants. It may slow growth initially, but weight and height generally stabilize over time.
⚠️
Behavioral and Cardiovascular Risks
Strattera may increase suicidal thoughts and affect heart rate and blood pressure in children, requiring close monitoring, especially early in treatment.
🩺
Need for Regular Monitoring
Regular checking of behavioral and physical health is important for children on Strattera to detect adverse effects early and ensure safety and effectiveness.
Highly Cited Studies
Long term Effects of Methylphenidate in Adults
Peer Reviewed Study 1
Two-Year Study Confirms Atomoxetine's Long-Term Safety and Efficacy in Adolescents
Peer Reviewed Study 2
Long-term safety and effectiveness of Strattera in young children with ADHD
Peer Reviewed Study 3
Review of Atomoxetine Safety in Long-Term ADHD Treatment
Background: Strattera's Long-Term Efficacy and Impact on Growth in Children with ADHD
Strattera (atomoxetine) has shown effectiveness in managing ADHD symptoms during long-term use. However, its effectiveness is generally lower compared to stimulants. Some studies suggest that Strattera can be well-tolerated for up to five years, though more high-quality, long-term research is needed.
Strattera has been associated with slowed growth in the first 9-12 months of treatment, but weight gain often rebounds over time. Height gain typically stabilizes after the first year.
Strattera has been associated with slowed growth in the first 9-12 months of treatment, but weight gain often rebounds over time. Height gain typically stabilizes after the first year.
“
Source Quotes:
Atomoxetine is less effective than stimulants but well tolerated.,Nonetheless, one concluded that medications continue to be effective and well-tolerated over at least 12 months, with a single study suggesting effectiveness for five years.
In general, the weight and height gain of pediatric patients treated with STRATTERA lags behind that predicted by normative population data for about the first 9-12 months of treatment.,After about 12 months, gain in height stabilizes, and at 3 years, patients treated with STRATTERA have gained 16.4 cm on average, 0.4 cm less than predicted by their baseline data.
Background: Behavioral and Cardiovascular Risks of Strattera
Strattera has been linked to behavioral changes, including an increased risk of suicidal thoughts in children and adolescents. This risk is higher in the early stages of treatment or when doses are adjusted.
Cardiovascular risks, including increased heart rate and blood pressure, have also been noted. These changes are generally mild but are important to monitor, especially in children with preexisting heart conditions.
Cardiovascular risks, including increased heart rate and blood pressure, have also been noted. These changes are generally mild but are important to monitor, especially in children with preexisting heart conditions.
“
Source Quotes:
Studies have shown that children and teenagers with attention-deficit hyperactivity disorder (ADHD) who take atomoxetine are more likely to think about killing themselves than children and teenagers with ADHD who do not take atomoxetine.
Pediatric placebo-controlled trials reported that STRATTERA-treated subjects experienced a mean increase in heart rate of about 6 beats/minute compared with placebo subjects.
Background: Importance of Regular Monitoring During Strattera Treatment
Regular monitoring is advised for children taking Strattera, especially at the beginning of treatment and during any dose changes. This includes checking behavioral and physical health to detect any adverse effects early.
Parents should maintain open communication with healthcare providers and report any changes in their child's behavior or health immediately.
Parents should maintain open communication with healthcare providers and report any changes in their child's behavior or health immediately.
“
Source Quotes:
Your child's doctor will want to see your child often while he or she is taking atomoxetine, especially at the beginning of his or her treatment.
All patients started on atomoxetine should have their vital signs monitored.
Peer Reviewed Study
Study: Two-Year Study Confirms Atomoxetine's Long-Term Safety and Efficacy in Adolescents
This meta-analysis pooled data from 13 studies to assess the long-term efficacy and safety of atomoxetine in adolescents aged 12 to 18 with ADHD. Over a two-year period, atomoxetine maintained its effectiveness without requiring dosage increases, and no new safety concerns were identified. The majority of subjects did not experience significant adverse effects, and the medication did not negatively impact growth, blood pressure, or heart rate.
A small percentage of subjects discontinued due to lack of effectiveness or adverse events, but the overall findings suggest sustained benefits for long-term use.
A small percentage of subjects discontinued due to lack of effectiveness or adverse events, but the overall findings suggest sustained benefits for long-term use.
author
Wilens TE, Newcorn JH, Kratochvil CJ, Gao H, Thomason CK, Rogers AK, Feldman PD, Levine LR
journal
J Pediatr
Date Published
2006 Jul
Peer Reviewed Study
Study: Long-term safety and effectiveness of Strattera in young children with ADHD
This meta-analysis combined data from 13 studies involving 6- and 7-year-olds with ADHD, treated with Strattera for over 2 years. Of the 272 participants, 97 were studied for the full 24 months, showing maintained effectiveness. Most children continued treatment, with only 4% discontinuing due to adverse events, which were generally minor. Growth impacts were observed early on but lessened over time. No clinically significant changes in vital signs or lab results were found.
author
Kratochvil CJ, Wilens TE, Greenhill LL, Gao H, Baker KD, Feldman PD, Gelowitz DL
journal
J Am Acad Child Adolesc Psychiatry
Date Published
2006 Aug

Peer Reviewed Study
Study: Review of Atomoxetine Safety in Long-Term ADHD Treatment
This abstract reviews safety data from 70 papers on atomoxetine, focusing on children and adolescents with ADHD. Key findings include a low hazard ratio for suicide-related events, no significant increase in aggression or hostility, and uncommon instances of psychosis and seizures. Cardiovascular effects were generally mild and reversible, and growth reductions appeared to recover over time.
Although rare cases of liver injury were noted, overall, atomoxetine was found to be safe for long-term use, with most adverse effects being uncommon and manageable.
Although rare cases of liver injury were noted, overall, atomoxetine was found to be safe for long-term use, with most adverse effects being uncommon and manageable.
author
Reed VA, Buitelaar JK, Anand E, Day KA, Treuer T, Upadhyaya HP, Coghill DR, Kryzhanovskaya LA, Savill NC
journal
CNS Drugs
Date Published
2016 Jul

Key Takeaways
Conclusions
Strattera (atomoxetine) has demonstrated efficacy in managing ADHD symptoms over the long-term in children and adolescents, though generally less effective than stimulant medications. Studies indicate it is well-tolerated for up to five years with vigilance in monitoring growth patterns to address any deviations.
Behavioral changes, including increased risk of suicidal thoughts, and mild cardiovascular risks like elevated heart rates have also been observed, emphasizing the need for consistent monitoring. Regular oversight by healthcare providers and open communication with parents is crucial to ensure safety during treatment.
Behavioral changes, including increased risk of suicidal thoughts, and mild cardiovascular risks like elevated heart rates have also been observed, emphasizing the need for consistent monitoring. Regular oversight by healthcare providers and open communication with parents is crucial to ensure safety during treatment.
Evidence Summary
Comparing Atomoxetine’s Safety and Side Effects in ADHD Treatment
Atomoxetine, a medication for ADHD, is highlighted here with a focus on its safety and side effects. The article compares atomoxetine's risk profile to other ADHD treatments, offering a detailed look at what patients may experience.
Side effects are an important consideration, and this content provides insights into how atomoxetine stacks up against alternative medications in terms of safety and tolerability.
Side effects are an important consideration, and this content provides insights into how atomoxetine stacks up against alternative medications in terms of safety and tolerability.
Evidence Summary
Atomoxetine: A Non-Stimulant Option for Managing ADHD
Atomoxetine is used to treat ADHD by targeting brain chemicals responsible for impulse control and hyperactivity. Taken as a capsule, it helps improve focus and reduce ADHD symptoms in children and adolescents.
This medication affects the brain's chemistry to manage symptoms, offering an alternative to stimulants for those seeking a different treatment option.
This medication affects the brain's chemistry to manage symptoms, offering an alternative to stimulants for those seeking a different treatment option.
Evidence Summary
Long-Term Impact of Strattera on ADHD Symptoms and Side Effects
The study tracks how long-term use of Strattera affects children with ADHD, focusing on changes in symptoms and potential side effects. It highlights both the safety and effectiveness of the medication over time.
The research carefully examines how the medication influences ADHD symptoms and any emerging side effects, aiming to provide a clearer picture of its overall impact on young patients.
The research carefully examines how the medication influences ADHD symptoms and any emerging side effects, aiming to provide a clearer picture of its overall impact on young patients.