Regardless of how you first heard of hydroxychloroquine, it seems like everyone is talking about it. Can this be the miracle drug that saves us from the pandemic?
At the moment, the evidence is still unclear about the effectiveness of hydroxychloroquine. However, without too many other options, many doctors have begun using hydroxychloroquine for their patients.
In this article, we will review the evidence for hydroxychloroquine.
What is Hydroxychloroquine?
Hydroxychloroquine (HCQ) is a prescription drug taken orally. It is approved by the FDA for the treatment of certain diseases, like malaria, arthritis, and lupus.
This year, the FDA has authorized HCQ for emergency use to treat patients with coronavirus. In a laboratory setting, the drug has shown activity against coronavirus.
Many clinical trials are currently underway to determine if HCQ is actually an effective treatment for coronavirus. As for now, doctors are reporting their experiences with the drug, their patient outcomes, and adverse effects.
The Wuhan Hydroxychloroquine Study
Study of 62 Patients in Wuhan
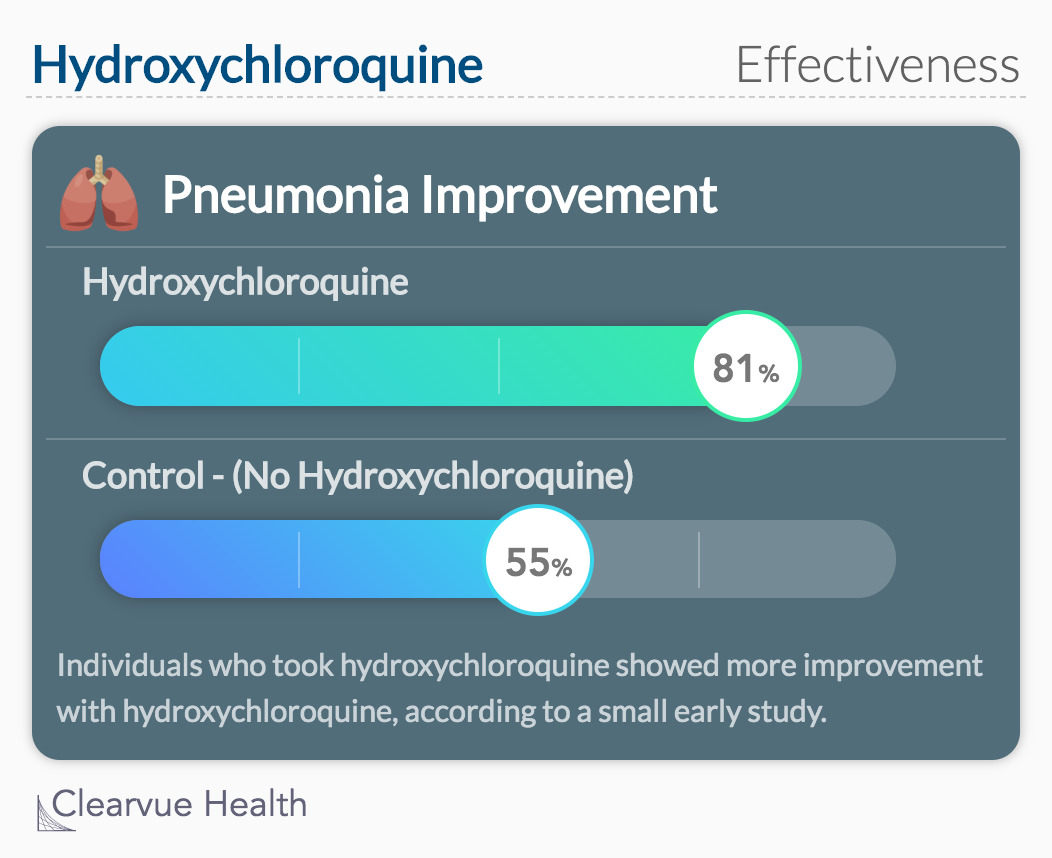
In Wuhan, China, clinicians reported on coronavirus treatment between February 4-28 of this year. 62 patients were assigned to a treatment group. Half of the patients received an additional 5-day HCQ treatment.
The study measured the patient’s time to recovery, alleviated symptoms, clinical characteristics, and test results over the study period. It also measured the percentage of people in each group who’s pneumonia improved.
Results
Over the study period, significantly more patients who took additional HCQ had improved pneumonia.
Fever and cough were alleviated slightly faster in the HCQ group, however, the difference between the groups was not significant.
Participants who took HCQ were alleviated of symptoms slightly quicker than patients in the control group. However, the difference between symptom alleviation days is too small to make any conclusions.
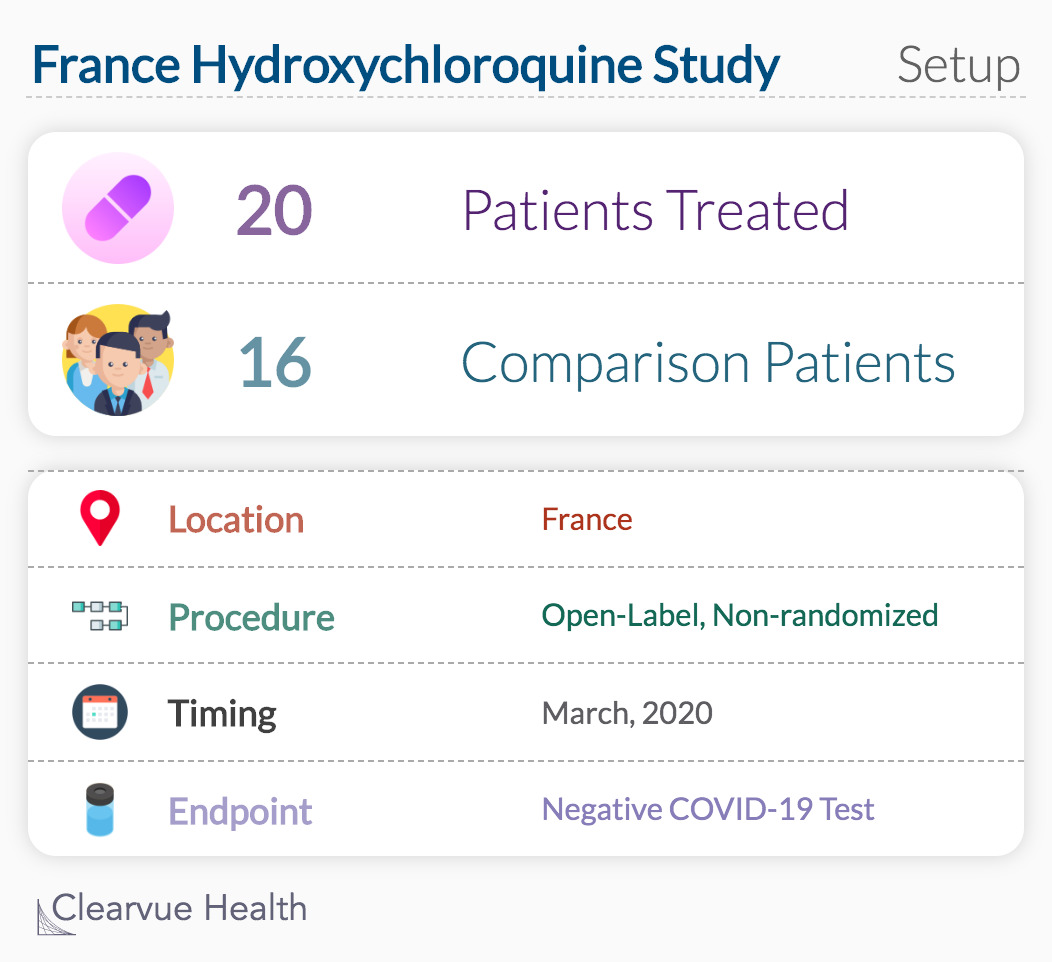
Study of 36 Patients in France
Shortly after the Wuhan study concluded, a hospital in France began treating patients with HCQ and azithromycin (AZ), a drug that treats infections such as bronchitis and pneumonia.
In the French hospital, 36 patients were put into treatment groups in early March 2020. Based on the doctor’s request, the patient received standard of care, HCQ, or HCQ plus azithromycin(AZ).
Standard of care is the expected treatment from a physician based on their knowledge, abilities, and resources. Being treated with the standard of care means that you are receiving appropriate and quality care based on available, evidence-based treatment options.
After six days, those treated with hydroxychloroquine showed promising improvement compared to similar patients not treated with hydroxychloroquine.
Of note, this was not a randomized controlled study, the gold standard of medical research.
France HydroxychloroquineResults
% with Positive PCR Results
6 days |
| Hydroxychloroquine (%) | 29 |
| Control (%) | 87 |
Yes, patients who took hydroxychloroquine recovered faster than the other treatment groups, but, it was a very small study. The sample size was even smaller than the Wuhan study.
There is a high likelihood that the recovery time of those six patients was due to chance, alone. Risk factors such as age, gender, substance use, and pre-existing conditions were not even between the groups and could have influenced their clinical outcomes. The who recovered may have been healthier in the first place or had less severe cases.
Time will tell
Studies like these quickly sparked the hype for HCQ as a potential treatment for coronavirus. The results were seen as a promising breakthrough. On the other hand, it is a scientist’s duty to be objective about these results and interpret them as accurately as possible. Yes, some patients have had success with HCQ, but that does not mean everyone will react the same way.
In addition, clinicians did not observe adverse events or side effects specific to HCQ in either study. This may be due to the same number of people and the short time they were observed. These events, which can be serious, are not to be ruled out. A larger sample is required to assess how likely a patient is to experience the side effects of the drugs.
Countries all around the world are using the drug and conducting large-scale clinical trials to evaluate its effectiveness. Studies like these are not meant to instruct doctors on what to do. Instead, it is meant to start a conversation about this particular course of treatment.