Effectiveness of Teplizumab in Delaying Diabetes

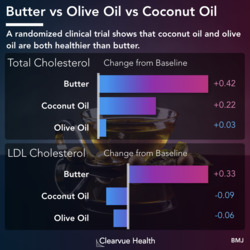
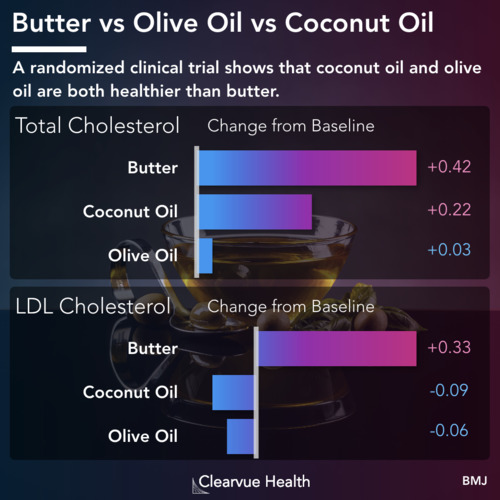
Figure 1: Teplizumab and Type 1 Diabetes. Patients who received the new treatment had a significantly lower risk of developing Type 1 Diabetes. Each line represents the proportion of patients who were free of Type 1 diabetes at each time point. The blue line represents patients who received the drug. The white line represents patients who received a placebo control. As you can see above, the blue line is much lower than the white light that every time point showing that the drugs were effective in reducing diabetes risk.
A new study provides hope that we may be able to prevent type one diabetes and some patients. Type one diabetes is caused by your immune system attacking the cells in the pancreas that are responsible for producing insulin.
This new therapy works to suppress the part of the immune cell that does this damage.
A new study in one of the most prestigious journals in medicine shows that doctors may have a new treatment that protects high-risk patients from developing Type 1 diabetes.
Doctors studied relatives of Type 1 Diabetes patients who had already developed early signs of Type 1 Diabetes. While the volunteer children were not diabetic, they were at a much higher risk of developing diabetes themselves. With current treatments, there is not much that we can do for these kids. A new medication offers some hope. As shown above, patients who received the new treatment had a significantly lower rate of developing Type 1 Diabetes over the 60 months they were followed. Those who did eventually develop Type 1 Diabetes had a significant delay, providing more years free of Type 1 Diabetes.
Source: An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes
Top Side Effects of Teplizumab

Figure 2: Top Side Effects of Teplizumab. The most common class of side effects were immune side effects. 75% of patients in the treatment group experienced immune side effects, compared to 6% of patients in the control group. Immune side effects were mostly transient lymphocyte losses, a common effect of immunotherapies. 36% of patients in the treatment group experienced skin side effects compared to 3% of the control group. Skin side effects were mostly rashes that got better on their own.
The downsides of this drug and immunotherapy, in general, are the side effects. The immune system is essential to good health. Any drug that acts on the immune system carries risks of significant side effects.
Generally, the drug was safe. The most common side effects were categorized as blood and immune symptoms. These were mostly cases of Lymphopenia. This is an expected symptom for this type of drug. The second most common type of symptom was skin-type side effects. Most of these were cases of rash that got better on their own without treatment.
Source: An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes
Immune Side Effects of Teplizumab

Figure 3: Lymphopenia Side Effects of Teplizumab. The chart above shows the immune cell counts during the course of the study. The white line represents the control patients, who did not receive the drug. The colored line represents the patients who receive the treatment. As you can see above, the patients who received the drug had a rapid decline in their immune cell count. Over time, the patients who received the drug saw their immune cell counts return to normal.
One side effect that many doctors were concerned about was the effects of the drug on the immune system. Because the drug functions by suppressing part of the immune system, doctors were concerned about whether patients with experience damage to their immune system.
As the data above shows, the effects of the drug on the immune system were temporary. Patients quickly regained the immune cells they had lost.
On the other hand, the magnitude of the effects was relatively small showing that we still have a long way to go when it comes to Type 1 Diabetes. Many of the kids who have received the treatment still ended up developing diabetes. With the rapid pace of innovation within immunotherapy, this may just be the first of many.
Source: An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes
+
+
Study Type - This study used a randomized controlled trial, which is the gold standard in medicine for evaluating new treatments. The study type is the best in ensuring that the effects seen in the study are due to the study drug.
+
Endpoints - Researchers used development of diabetes as the endpoint of the study. This was the correct and appropriate endpoint to use.
+
Sample Size - Researchers used a large enough study to find significant results.
-
Study Length - This study did not extend long enough to see the long term effects of this drug.
National Institutes of Health
The effects of the drug were greatest in the first year after it was given, when 41% of participants developed clinical diabetes, mainly in the placebo group. Many factors, including age, could have contributed to the ability of teplizumab to delay clinical disease, since at-risk children and adolescents are known to progress to type 1 diabetes faster than adults. Faster progression of type 1 diabetes is associated with a highly active immune system, which may explain the impact of immune system-modulating drugs like teplizumab.
Science Magazine
Though some researchers describe the study as a prevention trial, Herold hastens to point out that, strictly speaking, it was designed to test delay of disease onset, not prevention. Testing prevention could mean waiting for participants to die to confirm they stay diabetes free—something that’s obviously not feasible. Still, he and others wonder whether there’s a subset for whom true prevention is possible; it will take many years to find out.
NEJM
In conclusion, in our trial, a 2-week course of treatment with teplizumab delayed the diagnosis of clinical type 1 diabetes in high-risk participants.
Clearvue Health is not affiliated with above organizations. The information above is provided to highlight and link to useful further reading.