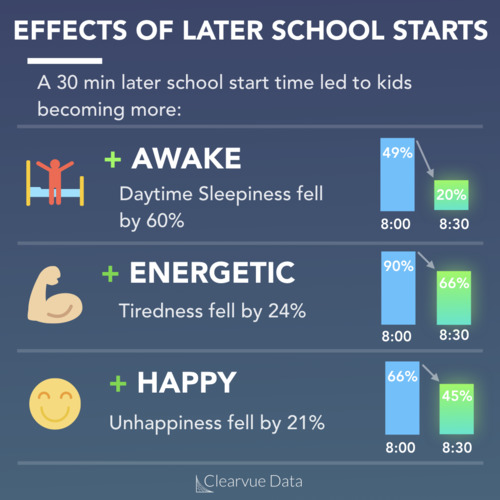
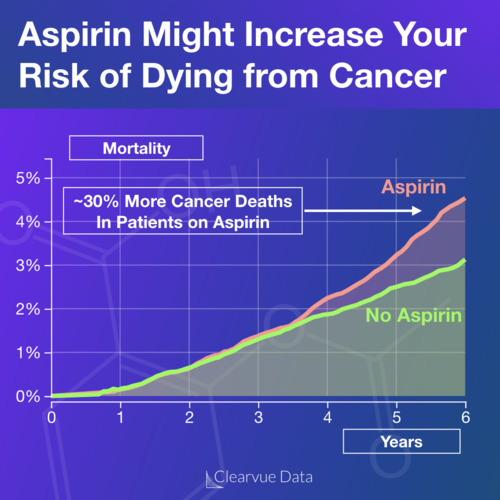
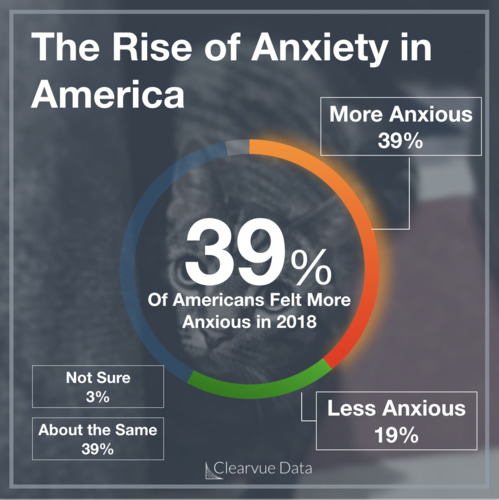
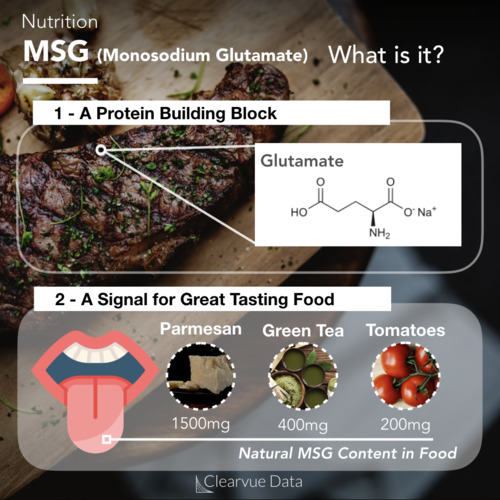
Peanut allergies are one of the most common allergies. Nearly 1.5% of children and teenagers report having a peanut allergy in the United States according to the latest data, though this can vary by study.
Accidental exposure to peanuts and peanut oil has caused numerous deaths in otherwise healthy kids.
Most concerning of all, this problem is only getting worse.
But, a new drug promises to change this trend.
AR101, made by the company Aimmune, exposes patients to a derived form of peanut protein in hopes that their bodies will become less sensitive to it.
Researchers recently published the results of their phase III trial, a trial designed to test the effectiveness of of the drug for FDA approval, in the New England Journal of Medicine.
In their study, researchers recruited 496 teenagers with known peanut allergies and randomly assigned them into two groups, one of took the new drug and the other which did not, in order to compare the effectiveness and side effects of the drug.

After 24 weeks of treatment and 24 weeks of lower dose maintenance therapy, patients were given 600mg of peanut protein, the protein equivalent of 5 pounds of roasted peanuts, to see whether they could eat the protein without side effects.
Their results showed that 67% of patients who received the treatment were able to tolerate the large dose of peanut protein at the end of the study, compared to only 4% of patients who did not receive the treatment:

However, the side effects were concerning. In order to tell which symptoms were the most common side effects of the drug, researchers compared the prevalence (how common) of each side effect in patients who took the drug and patients who did not:

More than half of the patients on the drug experienced abdominal pain (belly pain) and nearly half of all patients vomited during treatment period.
In fact, 4.3% if the patients in the trial had to withdraw due to very strong symptoms.
For patients with severe allergies that are impacting how they live their life, this drug offers a potential way to treat their allergy. As far as the results show, one 24 week course of treatment is very effective for up to 6 months after the initial treatment course, and therefore might possibly be effective for some patients for several years, though it has not been tested for that long.
However, the drug does carry some serious symptoms that may not justify the benefit for many patients. For the average patient, their current prevention method, epi-pens for example, and careful monitoring of their foods may be enough to prevent peanut allergies and would certainly not have the unpleasant side effects of AR101.